Then add the drying agent if there is a second layer on the bottom. The resulting solution is then centrifuged to separate the organic and aqueous layers.
Chm207 1c Docx Experiment 1c Extraction And Drying Of An Aqueous Solution Name Rawaida Binti Razali 2017257856 Group Members Muhammad Aidan Bin Course Hero
Reextract the organic layer from above two times with 30-ml portions of 1 M sodium hydroxide solution and combine these two aqueous extracts in another labeled flask.
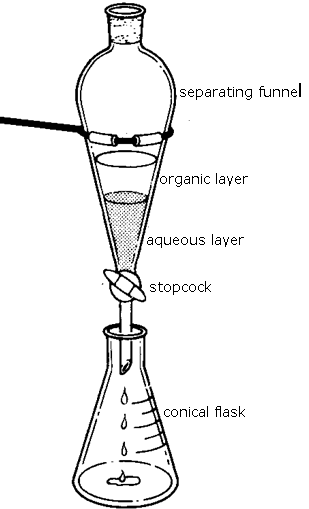
. This process is useful in extraction organic compounds such as organochloride and organophsophorus pesticides as well as. The aqueous solution of the given solute is taken in a separating funnel. In order to use a drying agent such a s sodium sulphate the drying.
Diethyl ether will place at the bottom layer because it is denser and have a higher molecular. Add a small amount enough to cover the bottom of the flask of anhydrous sodium sulfate Na 2 SO 4 to the ether solution to absorb any residual water. It is mixed with the desired organic solvent.
If it is all clumped together add more. Immiscible liquids do not dissolve in each other but they form layers when placed in the same separating funnel. Before drying agent is added the organic layer has to be separated from the aqueous layer.
This can then be recrystallized to yield pure solid neutral compound. This water must be removed before the required compound can be properly. A Add 20 mL of diethyl ether to the solution in the separatory funnel.
It is then allowed to remain undisturbed for some time. Use a pipet to carefully draw off the lower aqueous layer. An official website of the United States government.
In aqueous extraction some water. A drying agent such as magnesium sulfate can be used to further extract aqueous solvent from the organic solvent after extraction. Draw the apparatus needed for extraction.
- To learn the techniques of drying a dehydrated solution. 50-100 ml of dichloromethane. Drying organic solutions is the process of synthetizing and isolating an organic compound often results in an organic compound or solution contaminated with water.
The mixture of these two solutions give 2 phase layers which milky in colour when they are being separated. This process is done by injecting small amounts of an appropriate extraction solvent C 2 Cl 4 and a disperser solvent acetone into the aqueous solution. If the drying agent forms a clump at the bottom of the tube then more drying agent is needed.
Agent is just added to solution using a spatul a then the solution is stirred or swirled. After an organic solvent has been in contact with with an. Then the bottom layer of organic solution was drained into a dry conical flask and the top layer was pointed out to another conical flask.
B Place the plastic stopper NOT greased in the neck of the funnel. Mixture rapidly for 1-2 min to affect the acidbase reaction and subsequent extraction of A-into the aqueous layer. The ether solution using a drying procedure and the ether is evaporated to yield the solid neutral compound.
34C dichloromethane DCM bpt. In the experiment the percentage yield of toluene recovered is 32 and the percentage yield of ether recovered is 9. Extract this solution two times with 30-ml portions of 1 M hydrochloric acid.
Add drying agent until the organic solvent is sufficiently dried of aqueous solvent. 2020494116 COURSEDIPLOMA IN FOOD TECHNOLOGY CLASSAS1163A. Observe the drying agent.
Stop stirring and allow the two layers to separate. Usually water is used as aqueous phase and organic solvent as organic phase. The top one will be the aqueous solution which is the less dense one.
How do you estimate the anhydrous magnesium sulphate added is enough for the solvent in the experiment. EXTRACTION AND DRYING OF AN AQUEOUS SOLUTION NAMENURSYAFIQAH BINTI MOHAMAD FEZAR 020406-14-0264 STUDENT NUMBER. To learn the techniques of separation toluene from water and other.
The funnel is closed and its contents are shaken vigorously. Aqueous s olution it will be wet. Extraction is a technique that commonly used to separate an organic compound from an aqueous solution.
If the solution is tanslucent which means there is the presence of precipitate drying agent the solution is reasonable dry. The basic aqueous solution is neutralized with concentrated HCl to yield the carboxylic acid which because of its water insolubility precipitates out. The common organic solvents used in liquid-liquid extraction are diethyl ether ethoxyethane bpt.
DISCUSSION The result of extracted methylene chloride is as pure as before it is mixed with sodium bicarbonate. Will be transferred into the organic phase because of partial miscibility of the organic phase and water. In order to rem ove the dissolved wa ter a drying.
Extraction is a technique that commonly used to separate an organic compound from an aqueous solution. Aqueous extraction can be prepared with different methods it usually dried with frez dryer. Drying of Aqueous Solution 25 Liters spraying vessel Drying of water base solutions to micron-sized particles.
A liquid extraction involves two immiscible liquids. Extract the aqueous solution with diethyl ether in the following manner. 41C and ethyl acetate ethyl ethanoate bpt.
Meanwhile solid phase extraction dissolve or suspend the component in a liquid mixture and then separate it according to their physical and chemical properties. You can watch a short video that describes this drying process. Drying Patil is right.
Add a small amount of the solid drying agent directly to the organic solution. Solvent Extraction Methods. Dichloromethane is denser than water and forms the lower layer whereas diethyl ether and ethyl acetate float on water and are the upper layer.
Usually you will perform a wash with saturated sodium chloride solution to remove the bulk of the water before treating with an inorganic salt. For cytotoxic assays the use of fractions is more accurate than crude extract. It is because the parallax error happens during the experiment.
Be careful not to remove any of the upper ether layer. Heres how you know. Two phases will be separated.
Extraction Unit Soxhlet Extractor Unit Drying of Aqueous Solution Fractionation Column Unit Organic Solution Particle Formation Unit. Combine the aqueous extracts and set them aside in a labeled flask. Eject this solution from the pipet into a small beaker.
Esters And Esterification Chemistry Tutorial
Experiment 1c Extraction And Drying Of An Aqueous Solution Docx Experiment 1c Extraction And Drying Of An Aqueous Solution Objectives 1 2 To Learn Course Hero
Doc Inorganic Chem Mariam Hanani Ismail Academia Edu
4 8 Acid Base Extraction Chemistry Libretexts
Separating Components Of A Mixture By Extraction Youtube
4 6 Step By Step Procedures For Extractions Chemistry Libretexts
Lab 2 Docx Course Code Chm207 Experiment 3 Extraction And Drying Of An Aqueous Solution Name Intan Nurfarzana Binti Mohd Safini Student Id Course Hero
Pdf Effect Of Drying Method And Extraction Solvent On The Total Phenolics And Antioxidant Activity Of Cauliflower Brassica Oleracea L Extracts
Separating Components Of A Mixture By Extraction Youtube
Separation Of An Unknown Mixture
4 6 Step By Step Procedures For Extractions Chemistry Libretexts
Schematically Presentation Of Extraction Process For Precious Metals Download Scientific Diagram
Extraction Apparatus An Overview Sciencedirect Topics
4 6 Step By Step Procedures For Extractions Chemistry Libretexts
- gaya rambut undercut 2018
- sos yong tau fu
- kalendar kuda 2019 malaysia download
- villa for party in kl
- christmas dinner kl
- border kartun hitam putih doodle
- contoh surat peminjaman barang sekolah doc
- kang daniel wanna one
- yamaha rxz catalyzer biru putih
- produk putihkan ketiak di watson
- district meaning in malay
- kursus kahwin selangor 2019
- contoh surat permohonan membuat database
- henna putih pengantin terbaru
- disini lahirnya sebuah cinta lirik
- harga tayar kereta falken
- resepi ayam masak hitam berempah
- kad kredit islam terbaik
- baju kod kelabu pengantin
- gambar kasut perempuan hitam putih
